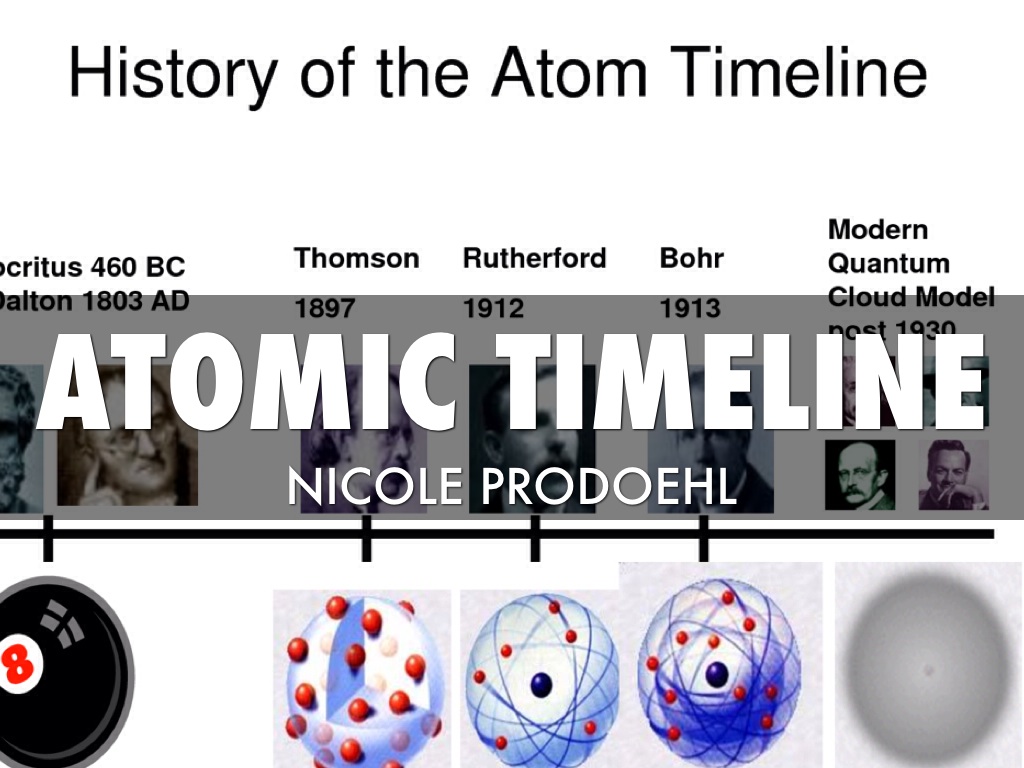
Atomic Timeline Nicole Prodoehl by Nicole Prodoehl
History of the Atom Project The atomic theory of matter is an excellent illustration of the process of science. Our understanding of the world around us is reshaped and refined with each scientific experiment. The first recorded idea of the atom comes from the ancient Greeks in the 400's B.C.

History Of Atoms Timeline boostermash
All matter is made up of atoms. This is something you learn right back at early chemistry classes. Despite this. our new ideas about what an atom is. is surp.
A History of the Atom Theories and Models
Matter is composed of exceedingly small particles called atoms. An atom is the smallest unit of an element that can participate in a chemical change. An element consists of only one type of atom, which has a mass that is characteristic of the element and is the same for all atoms of that element (Figure 2.1.1. 2.1. 1.

Timeline Of Atomic Models Stock Illustration Download Image Now Atom, Model Object
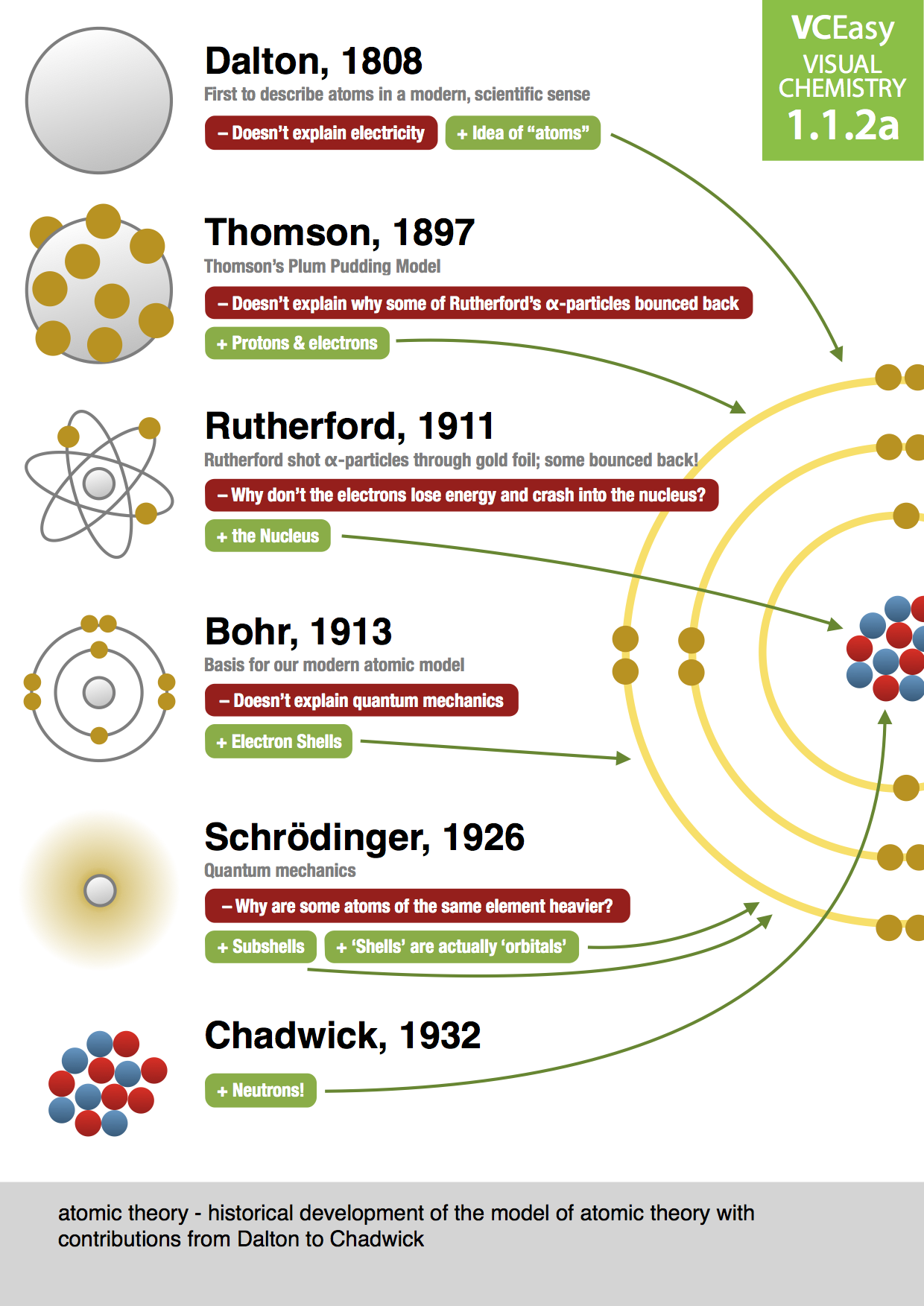
Dalton's atomic theory stated that different elements were made of different atoms, but did not explain what made the atoms different. The answer is that atoms are composed of subatomic particles, protons, neutron, and electrons, and the number of the protons and electrons in an atom defines the element it represents.
PPT History of the Atom Timeline PowerPoint Presentation, free download ID1169012
Early History of the Atom Matter is composed of indivisible building blocks. This idea was recorded as early as the fifth century BCE by Leucippus and Democritus. The Greeks called these particles atomos, meaning indivisible, and the modern word "atom" is derived from this term.

PPT The History of the Atom Timeline PowerPoint Presentation ID2061928
Leucippus of Miletus (5th century bce) is thought to have originated the atomic philosophy. His famous disciple, Democritus of Abdera, named the building blocks of matter atomos, meaning literally "indivisible," about 430 bce.

Atomic models timeline by Daenna González Issuu
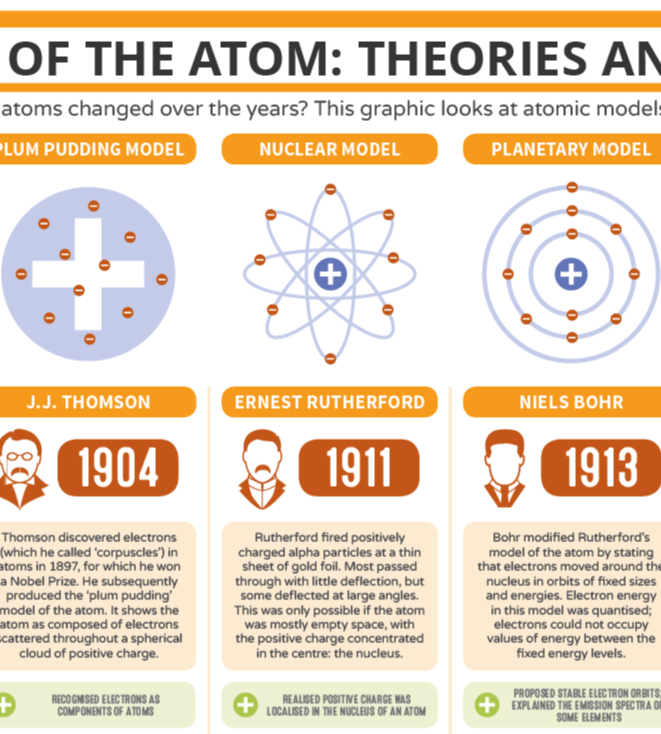
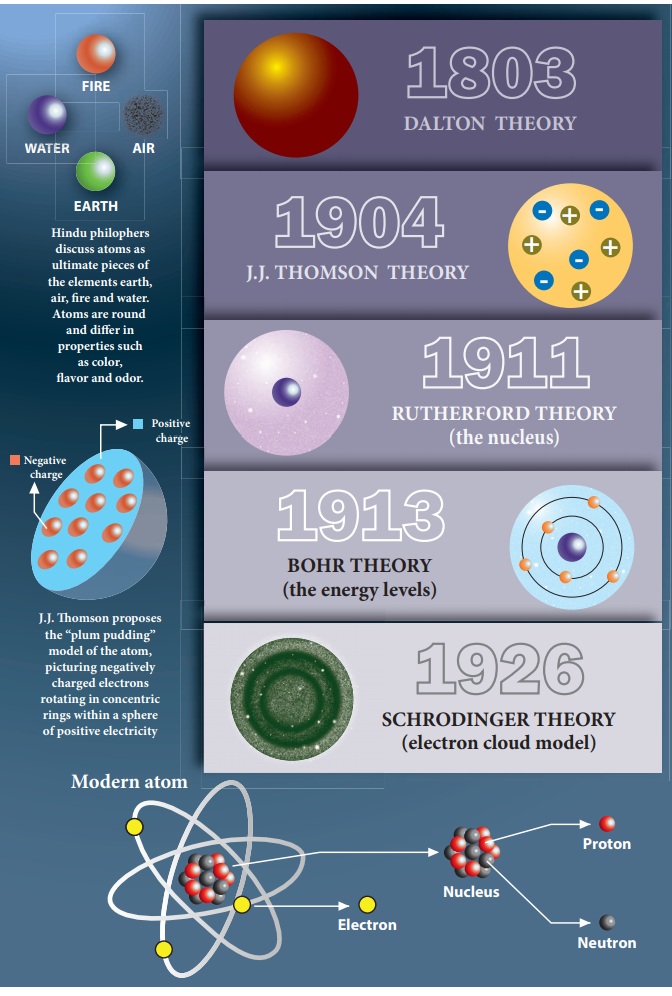
The structure of atoms is somehow related to electricity. (p.95) Discovered atoms have negative particles (electrons) using a cathode ray tube. Discovered electron's charge to mass ratio: 1.76 x 108 C/g. (p. 97-98) Thomson's Plum Pudding Model, 1900.
Evolution of Atomic Model 400 BC 2020 History of the atom Timeline, Atomic Theories YouTube
It wasn't until 1803 that the English chemist John Dalton started to develop a more scientific definition of the atom. He drew on the ideas of the Ancient Greeks in describing atoms as small, hard spheres that are indivisible, and that atoms of a given element are identical to each other.

Atomic theory on Pinterest Atoms, History and Chemistry
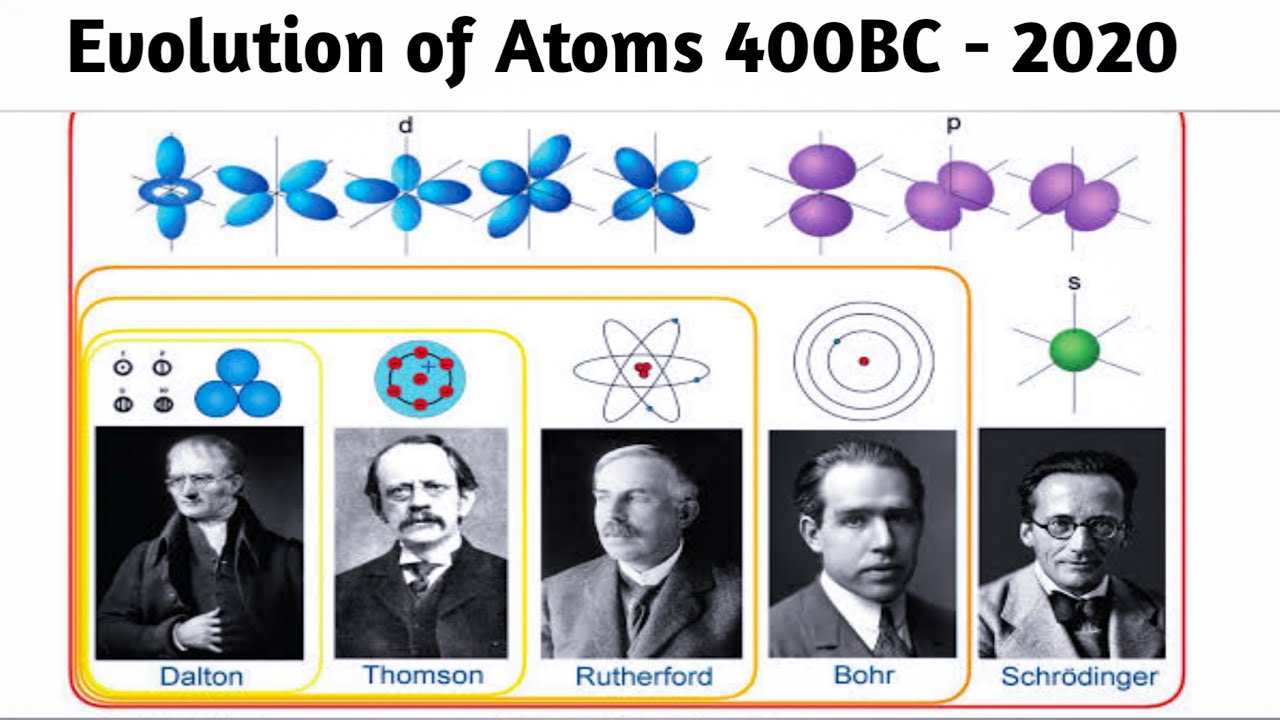
Atomic model, in physics, a model used to describe the structure and makeup of an atom. Atomic models have gone through many changes over time, evolving as necessary to fit experimental data. For a more in-depth discussion of the history of atomic models, see atom: development of atomic theory.
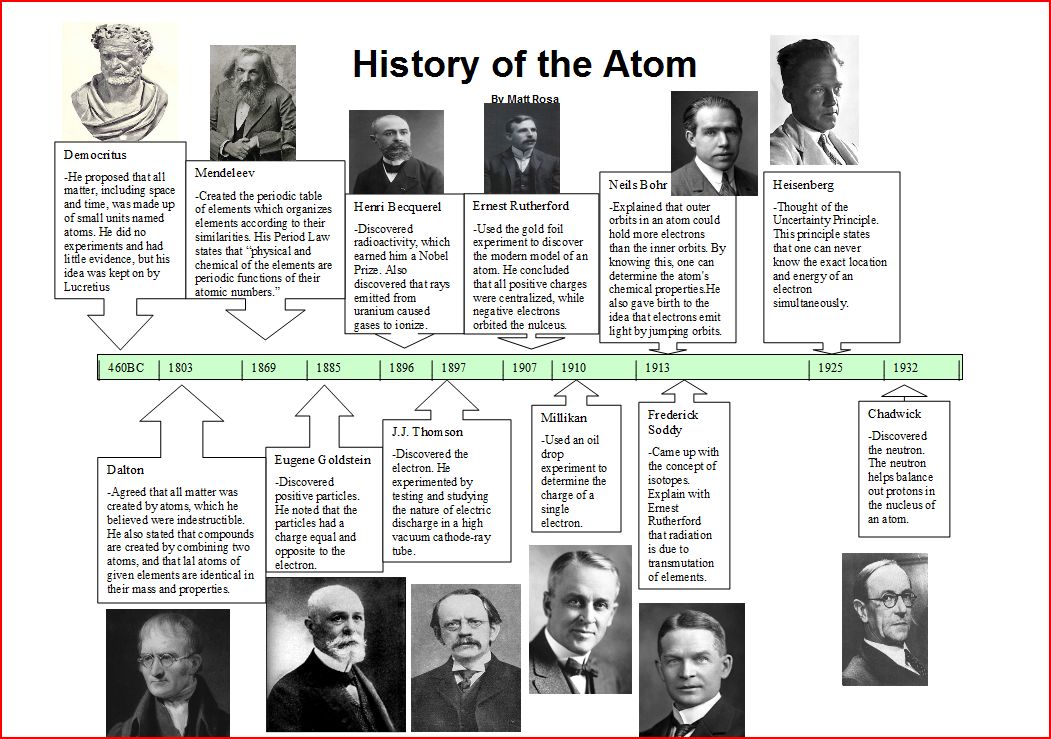
Slide 3
Matter is composed of exceedingly small particles called atoms. An atom is the smallest unit of an element that can participate in a chemical change. An element consists of only one type of atom, which has a mass that is characteristic of the element and is the same for all atoms of that element (Figure \(\PageIndex{1}\)). A macroscopic sample.

Atomic timeline Atom, Atomic theory, Timeline
Physics The Discovery of the Atom What would happen if we took a piece of paper and repeatedly cut it in half? Eventually, it would become impossible to continue with regular tools such as scissors, as the piece would simply be too small.
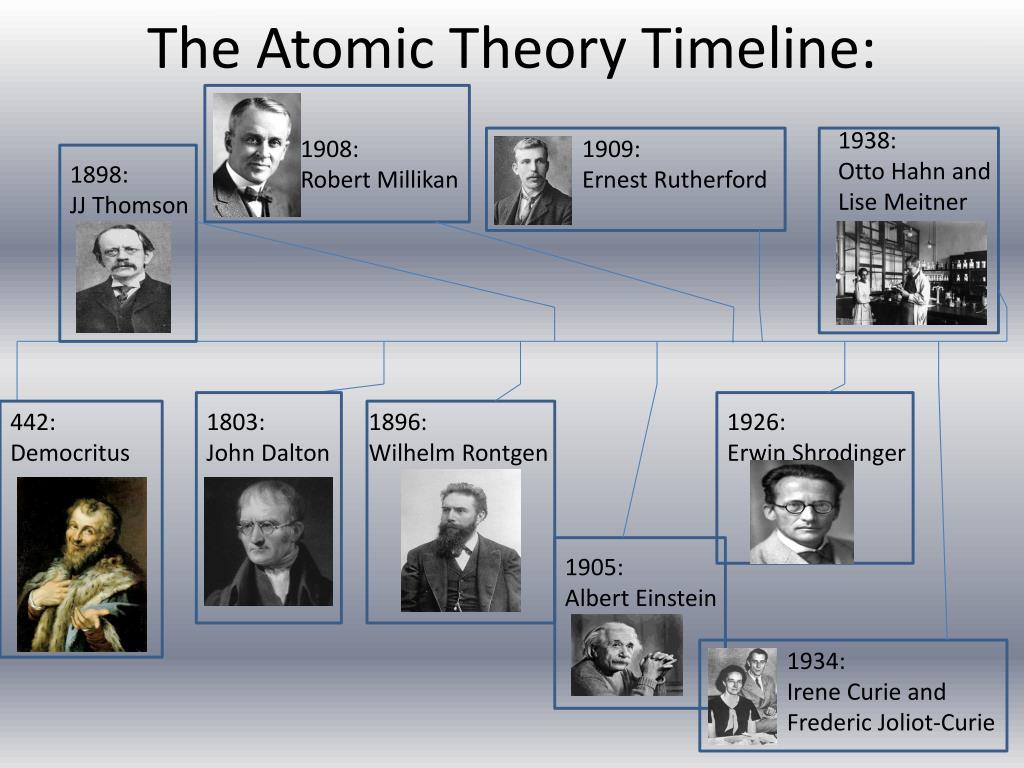
PPT The Atomic Theory Timeline PowerPoint Presentation, free download ID1854005
Center for History of Physics Teaching Guides The Evolution of Atomic Theory The Evolution of Atomic Theory J.J. Thomson and Ernest Rutherford conversing, Cavendish Laboratory, University of Cambridge. Photograph by D. Schoenberg, courtesy of AIP Emilio Segrè Visual Archives, Bainbridge Collection.

Atomic Theory Timeline Project A Visual History of the Atom Atomic theory, Science teaching
1803 John Dalton introduces atomic ideas into chemistry and states that matter is composed of atoms of different weights 1805 (approximate time) Thomas Young conducts the double-slit experiment with light 1811 Amedeo Avogadro claims that equal volumes of gases should contain equal numbers of molecules
Hazel Azaña
To see all my Chemistry videos, check outhttp://socratic.org/chemistryThis video is about the different ways that scientists have pictured the atoms over the.
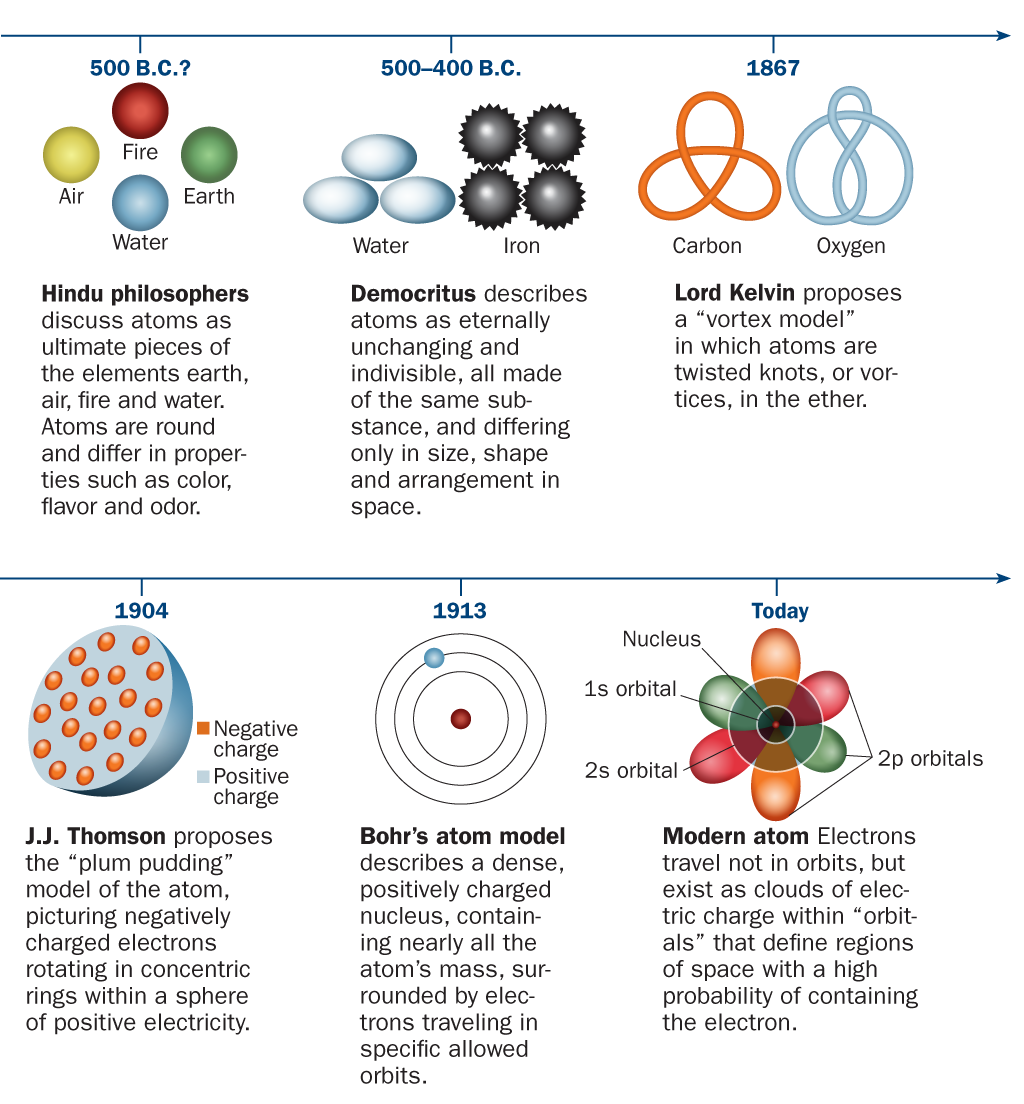
Science Visualized • A BRIEF TIMELINE OF ATOMIC THEORY The idea that...
1776 - 1844 He is from Cumberland, England. His atomic theory said that elements consisted of tiny particles called atoms. It states an element is one of a kind (aka pure) because all atoms of an element are identical. All the atoms that make up the element have the same mass. All elements are different from each other due to differing masses.
Evolution of idea of an atom Atomic Structure Term 1 Unit 4 7th Science
Greek philosopher Democritus. Atomic theory originated as a philosophical concept in ancient India and Greece. The word "atom" comes from the ancient Greek word atomos, which means indivisible. According to atomism, matter consists of discrete particles. However, the theory was one of many explanations for matter and wasn't based on empirical data.